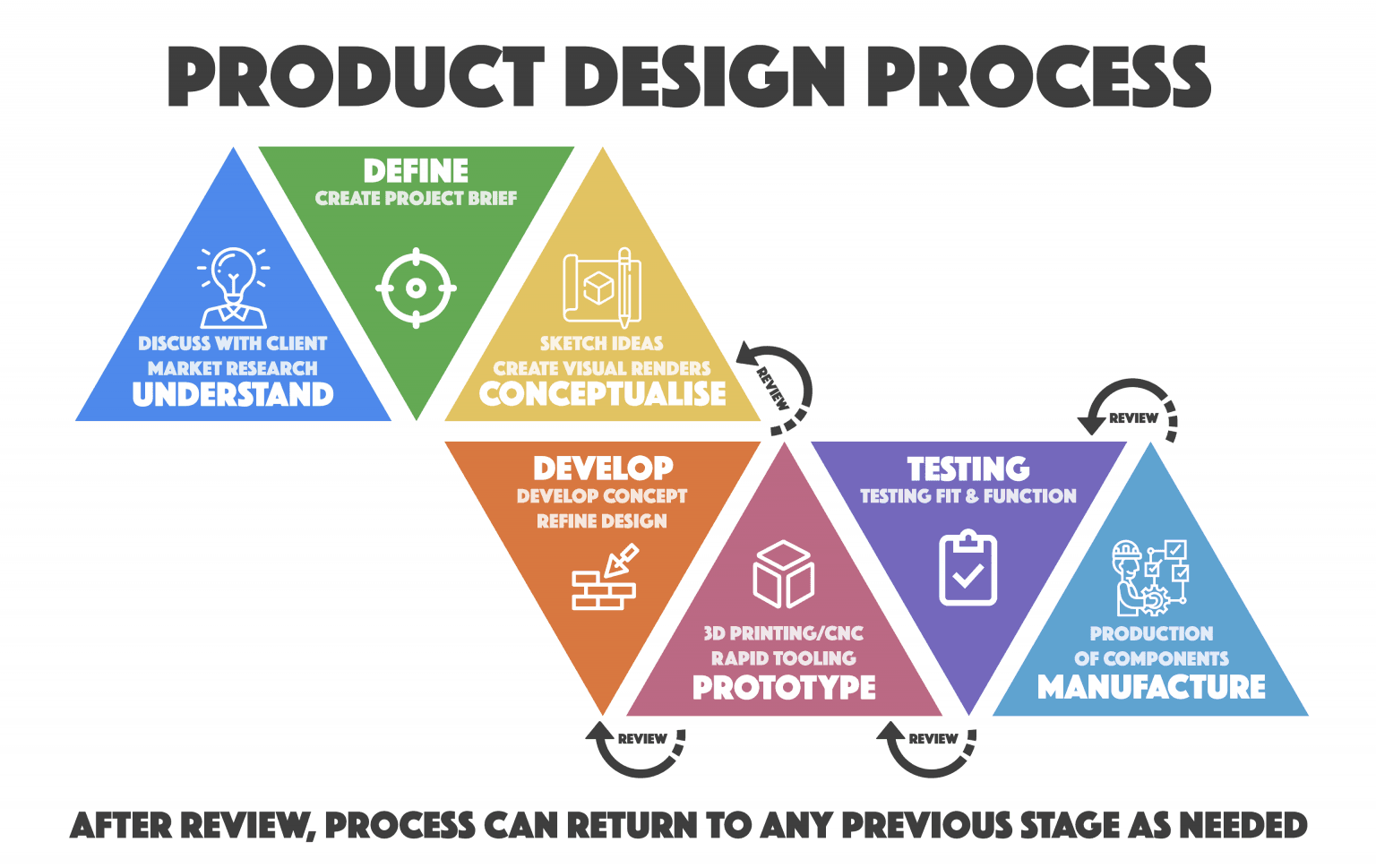
Our Three Step Process
November 19, 2024
How to Navigate Regulatory Hurdles in Product Development

Our Three Step Process
November 19, 2024
How to Navigate Regulatory Hurdles in Product Development
Discover strategies for effectively managing regulatory challenges in product development. This article explores practical approaches, real-world examples, and insights into integrating compliance into your process, highlighting NorthnStar's expertise in guiding clients through the complex regulatory landscape.
Why do some innovative products fail to reach the market despite their potential? Often, the answer lies in the complex web of regulatory requirements that can stall or even halt product development. Navigating these regulatory hurdles is crucial for turning great ideas into successful, compliant products. Let's explore how to effectively manage regulatory challenges in product development.
At NorthnStar, we recognize that regulatory compliance is a critical aspect of product development that can significantly impact time-to-market, costs, and overall success. Many clients have approached us facing difficulties in understanding and meeting regulatory requirements, leading to delays, increased expenses, and legal risks. Understanding the importance of proactive regulatory planning, we've developed strategies to help clients navigate these hurdles efficiently. This article delves into the complexities of regulatory compliance in product development, sharing insights and practical approaches to guide you through this challenging landscape.
The Importance of Navigating Regulatory Hurdles
Regulatory compliance ensures that products meet legal standards for safety, efficacy, and quality. Failing to comply can result in severe consequences, including fines, product recalls, or bans.
Why It Matters:
Market Access: Compliance is mandatory for entering and operating in target markets.
Risk Mitigation: Reduces legal liabilities and protects against financial penalties.
Consumer Safety: Ensures products are safe for use, building trust with customers.
Brand Reputation: Non-compliance can damage brand image and customer loyalty.
Competitive Advantage: Proactive compliance can accelerate time-to-market and outperform competitors.
Statistic:
According to a report by McKinsey & Company, companies that integrate regulatory strategies early in product development can reduce time-to-market by up to 20% and lower compliance costs by 30%.
Case Study: Successfully Navigating Regulatory Hurdles for a Medical Device
Client Challenge:
A medical technology startup aimed to develop an innovative wearable health monitor capable of real-time patient diagnostics. Despite having a groundbreaking product, they faced significant regulatory challenges:
Complex Approval Processes: Unfamiliarity with the FDA's stringent requirements for medical devices.
Resource Constraints: Limited budget and expertise to manage regulatory submissions.
Time Pressures: Investors expected rapid market entry to capitalize on the innovation.
Global Compliance: Plans to enter international markets added layers of regulatory complexity.
Recognizing that regulatory hurdles could derail their product launch, they approached NorthnStar for guidance.
Our Approach:
We set out to develop a comprehensive regulatory strategy integrated into the product development process, ensuring compliance without stifling innovation.
Actions Taken:
Regulatory Landscape Assessment:
Research and Analysis:
Identified all applicable regulations, including FDA classifications, European CE marking requirements, and other international standards.
Evaluated the device's risk classification to determine the appropriate regulatory pathway.
Regulatory Plan Development:
Created a detailed roadmap outlining submission requirements, timelines, and responsible parties.
Established milestones aligned with product development stages.
Design Controls Implementation:
Quality Management System (QMS):
Assisted in establishing a QMS compliant with ISO 13485 standards.
Integrated design controls to ensure traceability and documentation throughout development.
Risk Management:
Conducted risk analyses (e.g., FMEA) to identify potential hazards.
Developed mitigation strategies and incorporated them into the design.
Pre-Submission Engagement:
Regulatory Agency Interaction:
Scheduled pre-submission meetings with the FDA to clarify requirements and expectations.
Engaged with notified bodies in Europe for CE marking guidance.
Documentation Preparation:
Compiled necessary technical files, including design history, risk management reports, and clinical evaluation plans.
Clinical Evaluation and Testing:
Validation Studies:
Designed and conducted clinical trials to demonstrate safety and efficacy.
Collected data in compliance with Good Clinical Practice (GCP) guidelines.
Performance Testing:
Performed rigorous bench tests to validate device functionality under various conditions.
Regulatory Submission and Approval:
Regulatory Filing:
Prepared and submitted 510(k) premarket notification to the FDA.
Submitted technical documentation for CE marking in Europe.
Response Management:
Addressed questions and requests for additional information promptly.
Provided clarifications and supplementary data as needed.
Post-Market Surveillance Planning:
Monitoring Systems:
Established procedures for tracking product performance post-launch.
Developed reporting mechanisms for adverse events and corrective actions.
Regulatory Compliance Maintenance:
Scheduled regular audits and updates to ensure ongoing compliance with evolving regulations.
Outcome:
Regulatory Approval Achieved:
Successfully obtained FDA clearance and CE marking, allowing market entry in the U.S. and Europe.
Accelerated Time-to-Market:
Proactive planning reduced approval timelines by approximately six months.
Investor Confidence Boosted:
Demonstrated regulatory competence increased investor trust and secured additional funding.
Market Success:
The product launched successfully, receiving positive feedback for its innovation and reliability.
Brand Reputation Enhanced:
Compliance with high regulatory standards positioned the company as a trustworthy leader in medical technology.
Key Takeaways:
Early Integration: Incorporating regulatory considerations from the outset streamlines the development process.
Expert Guidance: Leveraging regulatory expertise mitigates risks and avoids costly mistakes.
Thorough Documentation: Comprehensive records and documentation are critical for successful submissions.
Active Engagement: Proactive communication with regulatory bodies facilitates smoother approval processes.
Ongoing Compliance: Regulatory responsibilities continue post-launch, requiring sustained attention.
Strategies for Navigating Regulatory Hurdles
1. Start with Regulatory Planning
Regulatory Roadmap:
Develop a clear plan outlining applicable regulations, timelines, and responsibilities.
Classification Determination:
Accurately classify your product to identify specific requirements and pathways.
2. Engage Regulatory Experts
Consultants and Legal Advisors:
Work with professionals specializing in regulatory affairs relevant to your industry.
Cross-Functional Teams:
Integrate regulatory experts into your development team for ongoing guidance.
3. Implement Robust Quality Management Systems
Compliance Standards:
Adopt QMS frameworks like ISO 9001 or ISO 13485, depending on your industry.
Design Controls:
Ensure traceability and documentation throughout the product lifecycle.
4. Prioritize Risk Management
Risk Assessments:
Conduct thorough analyses to identify and mitigate potential hazards.
Safety by Design:
Incorporate safety features and fail-safes into the product design.
5. Maintain Open Communication with Regulatory Bodies
Pre-Submission Meetings:
Seek feedback and clarification early in the process.
Transparency:
Provide clear and honest information to build trust with regulators.
6. Stay Informed on Regulatory Changes
Continuous Monitoring:
Keep abreast of evolving regulations and industry standards.
Training and Education:
Regularly update your team's knowledge on compliance requirements.
Challenges and How to Overcome Them
1. Complex and Evolving Regulations
Challenge: Regulations can be intricate and subject to change.
Solution: Engage dedicated regulatory professionals and invest in ongoing training.
2. Resource Limitations
Challenge: Small companies may lack the resources to manage compliance effectively.
Solution: Prioritize critical regulatory activities and consider outsourcing to specialists.
3. Global Compliance
Challenge: Navigating different regulations across multiple markets.
Solution: Develop region-specific strategies and collaborate with local experts.
4. Documentation Burden
Challenge: Extensive documentation can be time-consuming and overwhelming.
Solution: Implement efficient documentation systems and tools to streamline the process.
5. Fear of Regulatory Delays
Challenge: Concerns that engaging with regulators will slow down development.
Solution: Proactive communication often expedites approval by addressing issues early.
NorthnStar's Expertise in Navigating Regulatory Hurdles
At NorthnStar, we specialize in guiding clients through the complex regulatory landscape, ensuring compliance without compromising innovation.
Our Services Include:
Regulatory Strategy Development:
Crafting tailored plans that align with your product and market goals.
Compliance Consulting:
Providing expert advice on industry-specific regulations and standards.
Quality Management System Implementation:
Establishing QMS frameworks to support compliance and quality assurance.
Risk Management Support:
Conducting risk assessments and developing mitigation strategies.
Regulatory Submission Assistance:
Preparing documentation and managing interactions with regulatory bodies.
Training and Education:
Offering workshops and resources to enhance your team's regulatory knowledge.
Post-Market Compliance:
Supporting ongoing obligations, including surveillance and reporting.
Client Success Story:
Our collaboration with the medical technology startup demonstrates how proactive regulatory planning and expert guidance can turn potential hurdles into stepping stones. By integrating regulatory considerations into every stage of product development, we helped the client achieve faster market entry, reduce risks, and establish a strong foundation for future growth.
Conclusion
Navigating regulatory hurdles is a critical component of successful product development. By proactively addressing compliance requirements, engaging with experts, and integrating regulatory strategies into your process, you can turn potential obstacles into opportunities for competitive advantage.
At NorthnStar, we're committed to helping you navigate the complex regulatory landscape with confidence. Let us guide you in bringing your innovative products to market successfully and compliantly.
Ready to overcome regulatory challenges in your product development journey? Contact NorthnStar today, and let's pave the way to your success together.
Why do some innovative products fail to reach the market despite their potential? Often, the answer lies in the complex web of regulatory requirements that can stall or even halt product development. Navigating these regulatory hurdles is crucial for turning great ideas into successful, compliant products. Let's explore how to effectively manage regulatory challenges in product development.
At NorthnStar, we recognize that regulatory compliance is a critical aspect of product development that can significantly impact time-to-market, costs, and overall success. Many clients have approached us facing difficulties in understanding and meeting regulatory requirements, leading to delays, increased expenses, and legal risks. Understanding the importance of proactive regulatory planning, we've developed strategies to help clients navigate these hurdles efficiently. This article delves into the complexities of regulatory compliance in product development, sharing insights and practical approaches to guide you through this challenging landscape.
The Importance of Navigating Regulatory Hurdles
Regulatory compliance ensures that products meet legal standards for safety, efficacy, and quality. Failing to comply can result in severe consequences, including fines, product recalls, or bans.
Why It Matters:
Market Access: Compliance is mandatory for entering and operating in target markets.
Risk Mitigation: Reduces legal liabilities and protects against financial penalties.
Consumer Safety: Ensures products are safe for use, building trust with customers.
Brand Reputation: Non-compliance can damage brand image and customer loyalty.
Competitive Advantage: Proactive compliance can accelerate time-to-market and outperform competitors.
Statistic:
According to a report by McKinsey & Company, companies that integrate regulatory strategies early in product development can reduce time-to-market by up to 20% and lower compliance costs by 30%.
Case Study: Successfully Navigating Regulatory Hurdles for a Medical Device
Client Challenge:
A medical technology startup aimed to develop an innovative wearable health monitor capable of real-time patient diagnostics. Despite having a groundbreaking product, they faced significant regulatory challenges:
Complex Approval Processes: Unfamiliarity with the FDA's stringent requirements for medical devices.
Resource Constraints: Limited budget and expertise to manage regulatory submissions.
Time Pressures: Investors expected rapid market entry to capitalize on the innovation.
Global Compliance: Plans to enter international markets added layers of regulatory complexity.
Recognizing that regulatory hurdles could derail their product launch, they approached NorthnStar for guidance.
Our Approach:
We set out to develop a comprehensive regulatory strategy integrated into the product development process, ensuring compliance without stifling innovation.
Actions Taken:
Regulatory Landscape Assessment:
Research and Analysis:
Identified all applicable regulations, including FDA classifications, European CE marking requirements, and other international standards.
Evaluated the device's risk classification to determine the appropriate regulatory pathway.
Regulatory Plan Development:
Created a detailed roadmap outlining submission requirements, timelines, and responsible parties.
Established milestones aligned with product development stages.
Design Controls Implementation:
Quality Management System (QMS):
Assisted in establishing a QMS compliant with ISO 13485 standards.
Integrated design controls to ensure traceability and documentation throughout development.
Risk Management:
Conducted risk analyses (e.g., FMEA) to identify potential hazards.
Developed mitigation strategies and incorporated them into the design.
Pre-Submission Engagement:
Regulatory Agency Interaction:
Scheduled pre-submission meetings with the FDA to clarify requirements and expectations.
Engaged with notified bodies in Europe for CE marking guidance.
Documentation Preparation:
Compiled necessary technical files, including design history, risk management reports, and clinical evaluation plans.
Clinical Evaluation and Testing:
Validation Studies:
Designed and conducted clinical trials to demonstrate safety and efficacy.
Collected data in compliance with Good Clinical Practice (GCP) guidelines.
Performance Testing:
Performed rigorous bench tests to validate device functionality under various conditions.
Regulatory Submission and Approval:
Regulatory Filing:
Prepared and submitted 510(k) premarket notification to the FDA.
Submitted technical documentation for CE marking in Europe.
Response Management:
Addressed questions and requests for additional information promptly.
Provided clarifications and supplementary data as needed.
Post-Market Surveillance Planning:
Monitoring Systems:
Established procedures for tracking product performance post-launch.
Developed reporting mechanisms for adverse events and corrective actions.
Regulatory Compliance Maintenance:
Scheduled regular audits and updates to ensure ongoing compliance with evolving regulations.
Outcome:
Regulatory Approval Achieved:
Successfully obtained FDA clearance and CE marking, allowing market entry in the U.S. and Europe.
Accelerated Time-to-Market:
Proactive planning reduced approval timelines by approximately six months.
Investor Confidence Boosted:
Demonstrated regulatory competence increased investor trust and secured additional funding.
Market Success:
The product launched successfully, receiving positive feedback for its innovation and reliability.
Brand Reputation Enhanced:
Compliance with high regulatory standards positioned the company as a trustworthy leader in medical technology.
Key Takeaways:
Early Integration: Incorporating regulatory considerations from the outset streamlines the development process.
Expert Guidance: Leveraging regulatory expertise mitigates risks and avoids costly mistakes.
Thorough Documentation: Comprehensive records and documentation are critical for successful submissions.
Active Engagement: Proactive communication with regulatory bodies facilitates smoother approval processes.
Ongoing Compliance: Regulatory responsibilities continue post-launch, requiring sustained attention.
Strategies for Navigating Regulatory Hurdles
1. Start with Regulatory Planning
Regulatory Roadmap:
Develop a clear plan outlining applicable regulations, timelines, and responsibilities.
Classification Determination:
Accurately classify your product to identify specific requirements and pathways.
2. Engage Regulatory Experts
Consultants and Legal Advisors:
Work with professionals specializing in regulatory affairs relevant to your industry.
Cross-Functional Teams:
Integrate regulatory experts into your development team for ongoing guidance.
3. Implement Robust Quality Management Systems
Compliance Standards:
Adopt QMS frameworks like ISO 9001 or ISO 13485, depending on your industry.
Design Controls:
Ensure traceability and documentation throughout the product lifecycle.
4. Prioritize Risk Management
Risk Assessments:
Conduct thorough analyses to identify and mitigate potential hazards.
Safety by Design:
Incorporate safety features and fail-safes into the product design.
5. Maintain Open Communication with Regulatory Bodies
Pre-Submission Meetings:
Seek feedback and clarification early in the process.
Transparency:
Provide clear and honest information to build trust with regulators.
6. Stay Informed on Regulatory Changes
Continuous Monitoring:
Keep abreast of evolving regulations and industry standards.
Training and Education:
Regularly update your team's knowledge on compliance requirements.
Challenges and How to Overcome Them
1. Complex and Evolving Regulations
Challenge: Regulations can be intricate and subject to change.
Solution: Engage dedicated regulatory professionals and invest in ongoing training.
2. Resource Limitations
Challenge: Small companies may lack the resources to manage compliance effectively.
Solution: Prioritize critical regulatory activities and consider outsourcing to specialists.
3. Global Compliance
Challenge: Navigating different regulations across multiple markets.
Solution: Develop region-specific strategies and collaborate with local experts.
4. Documentation Burden
Challenge: Extensive documentation can be time-consuming and overwhelming.
Solution: Implement efficient documentation systems and tools to streamline the process.
5. Fear of Regulatory Delays
Challenge: Concerns that engaging with regulators will slow down development.
Solution: Proactive communication often expedites approval by addressing issues early.
NorthnStar's Expertise in Navigating Regulatory Hurdles
At NorthnStar, we specialize in guiding clients through the complex regulatory landscape, ensuring compliance without compromising innovation.
Our Services Include:
Regulatory Strategy Development:
Crafting tailored plans that align with your product and market goals.
Compliance Consulting:
Providing expert advice on industry-specific regulations and standards.
Quality Management System Implementation:
Establishing QMS frameworks to support compliance and quality assurance.
Risk Management Support:
Conducting risk assessments and developing mitigation strategies.
Regulatory Submission Assistance:
Preparing documentation and managing interactions with regulatory bodies.
Training and Education:
Offering workshops and resources to enhance your team's regulatory knowledge.
Post-Market Compliance:
Supporting ongoing obligations, including surveillance and reporting.
Client Success Story:
Our collaboration with the medical technology startup demonstrates how proactive regulatory planning and expert guidance can turn potential hurdles into stepping stones. By integrating regulatory considerations into every stage of product development, we helped the client achieve faster market entry, reduce risks, and establish a strong foundation for future growth.
Conclusion
Navigating regulatory hurdles is a critical component of successful product development. By proactively addressing compliance requirements, engaging with experts, and integrating regulatory strategies into your process, you can turn potential obstacles into opportunities for competitive advantage.
At NorthnStar, we're committed to helping you navigate the complex regulatory landscape with confidence. Let us guide you in bringing your innovative products to market successfully and compliantly.
Ready to overcome regulatory challenges in your product development journey? Contact NorthnStar today, and let's pave the way to your success together.
Discover strategies for effectively managing regulatory challenges in product development. This article explores practical approaches, real-world examples, and insights into integrating compliance into your process, highlighting NorthnStar's expertise in guiding clients through the complex regulatory landscape.
Why do some innovative products fail to reach the market despite their potential? Often, the answer lies in the complex web of regulatory requirements that can stall or even halt product development. Navigating these regulatory hurdles is crucial for turning great ideas into successful, compliant products. Let's explore how to effectively manage regulatory challenges in product development.
At NorthnStar, we recognize that regulatory compliance is a critical aspect of product development that can significantly impact time-to-market, costs, and overall success. Many clients have approached us facing difficulties in understanding and meeting regulatory requirements, leading to delays, increased expenses, and legal risks. Understanding the importance of proactive regulatory planning, we've developed strategies to help clients navigate these hurdles efficiently. This article delves into the complexities of regulatory compliance in product development, sharing insights and practical approaches to guide you through this challenging landscape.
The Importance of Navigating Regulatory Hurdles
Regulatory compliance ensures that products meet legal standards for safety, efficacy, and quality. Failing to comply can result in severe consequences, including fines, product recalls, or bans.
Why It Matters:
Market Access: Compliance is mandatory for entering and operating in target markets.
Risk Mitigation: Reduces legal liabilities and protects against financial penalties.
Consumer Safety: Ensures products are safe for use, building trust with customers.
Brand Reputation: Non-compliance can damage brand image and customer loyalty.
Competitive Advantage: Proactive compliance can accelerate time-to-market and outperform competitors.
Statistic:
According to a report by McKinsey & Company, companies that integrate regulatory strategies early in product development can reduce time-to-market by up to 20% and lower compliance costs by 30%.
Case Study: Successfully Navigating Regulatory Hurdles for a Medical Device
Client Challenge:
A medical technology startup aimed to develop an innovative wearable health monitor capable of real-time patient diagnostics. Despite having a groundbreaking product, they faced significant regulatory challenges:
Complex Approval Processes: Unfamiliarity with the FDA's stringent requirements for medical devices.
Resource Constraints: Limited budget and expertise to manage regulatory submissions.
Time Pressures: Investors expected rapid market entry to capitalize on the innovation.
Global Compliance: Plans to enter international markets added layers of regulatory complexity.
Recognizing that regulatory hurdles could derail their product launch, they approached NorthnStar for guidance.
Our Approach:
We set out to develop a comprehensive regulatory strategy integrated into the product development process, ensuring compliance without stifling innovation.
Actions Taken:
Regulatory Landscape Assessment:
Research and Analysis:
Identified all applicable regulations, including FDA classifications, European CE marking requirements, and other international standards.
Evaluated the device's risk classification to determine the appropriate regulatory pathway.
Regulatory Plan Development:
Created a detailed roadmap outlining submission requirements, timelines, and responsible parties.
Established milestones aligned with product development stages.
Design Controls Implementation:
Quality Management System (QMS):
Assisted in establishing a QMS compliant with ISO 13485 standards.
Integrated design controls to ensure traceability and documentation throughout development.
Risk Management:
Conducted risk analyses (e.g., FMEA) to identify potential hazards.
Developed mitigation strategies and incorporated them into the design.
Pre-Submission Engagement:
Regulatory Agency Interaction:
Scheduled pre-submission meetings with the FDA to clarify requirements and expectations.
Engaged with notified bodies in Europe for CE marking guidance.
Documentation Preparation:
Compiled necessary technical files, including design history, risk management reports, and clinical evaluation plans.
Clinical Evaluation and Testing:
Validation Studies:
Designed and conducted clinical trials to demonstrate safety and efficacy.
Collected data in compliance with Good Clinical Practice (GCP) guidelines.
Performance Testing:
Performed rigorous bench tests to validate device functionality under various conditions.
Regulatory Submission and Approval:
Regulatory Filing:
Prepared and submitted 510(k) premarket notification to the FDA.
Submitted technical documentation for CE marking in Europe.
Response Management:
Addressed questions and requests for additional information promptly.
Provided clarifications and supplementary data as needed.
Post-Market Surveillance Planning:
Monitoring Systems:
Established procedures for tracking product performance post-launch.
Developed reporting mechanisms for adverse events and corrective actions.
Regulatory Compliance Maintenance:
Scheduled regular audits and updates to ensure ongoing compliance with evolving regulations.
Outcome:
Regulatory Approval Achieved:
Successfully obtained FDA clearance and CE marking, allowing market entry in the U.S. and Europe.
Accelerated Time-to-Market:
Proactive planning reduced approval timelines by approximately six months.
Investor Confidence Boosted:
Demonstrated regulatory competence increased investor trust and secured additional funding.
Market Success:
The product launched successfully, receiving positive feedback for its innovation and reliability.
Brand Reputation Enhanced:
Compliance with high regulatory standards positioned the company as a trustworthy leader in medical technology.
Key Takeaways:
Early Integration: Incorporating regulatory considerations from the outset streamlines the development process.
Expert Guidance: Leveraging regulatory expertise mitigates risks and avoids costly mistakes.
Thorough Documentation: Comprehensive records and documentation are critical for successful submissions.
Active Engagement: Proactive communication with regulatory bodies facilitates smoother approval processes.
Ongoing Compliance: Regulatory responsibilities continue post-launch, requiring sustained attention.
Strategies for Navigating Regulatory Hurdles
1. Start with Regulatory Planning
Regulatory Roadmap:
Develop a clear plan outlining applicable regulations, timelines, and responsibilities.
Classification Determination:
Accurately classify your product to identify specific requirements and pathways.
2. Engage Regulatory Experts
Consultants and Legal Advisors:
Work with professionals specializing in regulatory affairs relevant to your industry.
Cross-Functional Teams:
Integrate regulatory experts into your development team for ongoing guidance.
3. Implement Robust Quality Management Systems
Compliance Standards:
Adopt QMS frameworks like ISO 9001 or ISO 13485, depending on your industry.
Design Controls:
Ensure traceability and documentation throughout the product lifecycle.
4. Prioritize Risk Management
Risk Assessments:
Conduct thorough analyses to identify and mitigate potential hazards.
Safety by Design:
Incorporate safety features and fail-safes into the product design.
5. Maintain Open Communication with Regulatory Bodies
Pre-Submission Meetings:
Seek feedback and clarification early in the process.
Transparency:
Provide clear and honest information to build trust with regulators.
6. Stay Informed on Regulatory Changes
Continuous Monitoring:
Keep abreast of evolving regulations and industry standards.
Training and Education:
Regularly update your team's knowledge on compliance requirements.
Challenges and How to Overcome Them
1. Complex and Evolving Regulations
Challenge: Regulations can be intricate and subject to change.
Solution: Engage dedicated regulatory professionals and invest in ongoing training.
2. Resource Limitations
Challenge: Small companies may lack the resources to manage compliance effectively.
Solution: Prioritize critical regulatory activities and consider outsourcing to specialists.
3. Global Compliance
Challenge: Navigating different regulations across multiple markets.
Solution: Develop region-specific strategies and collaborate with local experts.
4. Documentation Burden
Challenge: Extensive documentation can be time-consuming and overwhelming.
Solution: Implement efficient documentation systems and tools to streamline the process.
5. Fear of Regulatory Delays
Challenge: Concerns that engaging with regulators will slow down development.
Solution: Proactive communication often expedites approval by addressing issues early.
NorthnStar's Expertise in Navigating Regulatory Hurdles
At NorthnStar, we specialize in guiding clients through the complex regulatory landscape, ensuring compliance without compromising innovation.
Our Services Include:
Regulatory Strategy Development:
Crafting tailored plans that align with your product and market goals.
Compliance Consulting:
Providing expert advice on industry-specific regulations and standards.
Quality Management System Implementation:
Establishing QMS frameworks to support compliance and quality assurance.
Risk Management Support:
Conducting risk assessments and developing mitigation strategies.
Regulatory Submission Assistance:
Preparing documentation and managing interactions with regulatory bodies.
Training and Education:
Offering workshops and resources to enhance your team's regulatory knowledge.
Post-Market Compliance:
Supporting ongoing obligations, including surveillance and reporting.
Client Success Story:
Our collaboration with the medical technology startup demonstrates how proactive regulatory planning and expert guidance can turn potential hurdles into stepping stones. By integrating regulatory considerations into every stage of product development, we helped the client achieve faster market entry, reduce risks, and establish a strong foundation for future growth.
Conclusion
Navigating regulatory hurdles is a critical component of successful product development. By proactively addressing compliance requirements, engaging with experts, and integrating regulatory strategies into your process, you can turn potential obstacles into opportunities for competitive advantage.
At NorthnStar, we're committed to helping you navigate the complex regulatory landscape with confidence. Let us guide you in bringing your innovative products to market successfully and compliantly.
Ready to overcome regulatory challenges in your product development journey? Contact NorthnStar today, and let's pave the way to your success together.
Other Blogs
Other Blogs
Check our other project Blogs with useful insight and information for your businesses
Other Blogs
Other Blogs
Check our other project Blogs with useful insight and information for your businesses
Other Blogs
Other Blogs
Check our other project Blogs with useful insight and information for your businesses